More Effective Treatments and a Cure for JM
Medical advances and improved treatments for any disease require scientific research that builds upon itself year after year to achieve better long-term outcomes for patients.

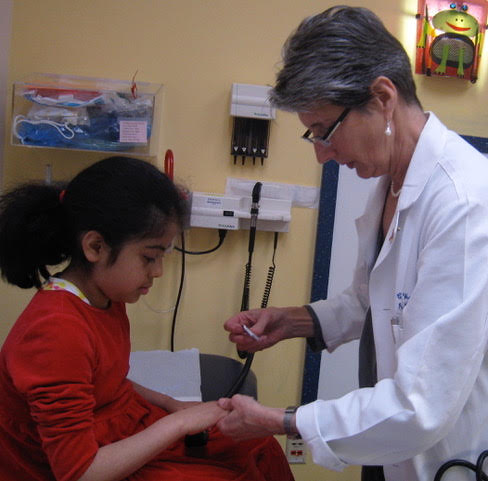
The Importance of Juvenile Myositis Research
Cure JM was founded 20 years ago by a small group of volunteers wanting to change the world for children with a rare disease few had ever heard of—juvenile myositis.
At the time, very little was known about JM, what caused it, or how to effectively treat it …
Key Research Outcomes
Multiple clinical trials are in development for more effective treatments with fewer side effects
$27 million invested in research, making Cure JM the largest funder of JM research on the planet
More than $5 million invested in current research projects
Research Updates
Cure JM is proud to offer grant funding for clinicians and researchers who are working to improve juvenile myositis care and advance research. Cure JM is also working diligently to find a cure for JM by working with scientists and doctors on new research every day.

Research Achievements
Juvenile myositis research is changing the world for our patients. Children are doing better than ever, and we are just getting started!
Cure JM’s Community Advisory Board plays a vital role in representing JM families from diverse backgrounds to help support this cutting edge research project. Cure JM CAB members are the link between the greater JM community and the project’s research team, working together to ensure that the needs of the entire JM community are understood and addressed.
