A clinical trial treating patients with juvenile myositis with the drug abatacept resulted in lower disease activity and clinically significant responses in most patients. The trial was conducted at the Cure JM Center of Excellence at George Washington University, and preliminary results were presented at the Global Conference on Myositis.
Abatacept was generally well tolerated with minimal side effects, and the preliminary data suggest that abatacept may be beneficial in the treatment of hard-to-treat JM patients. Importantly, patients using abatacept improved at the same time prednisone dosage was reduced.
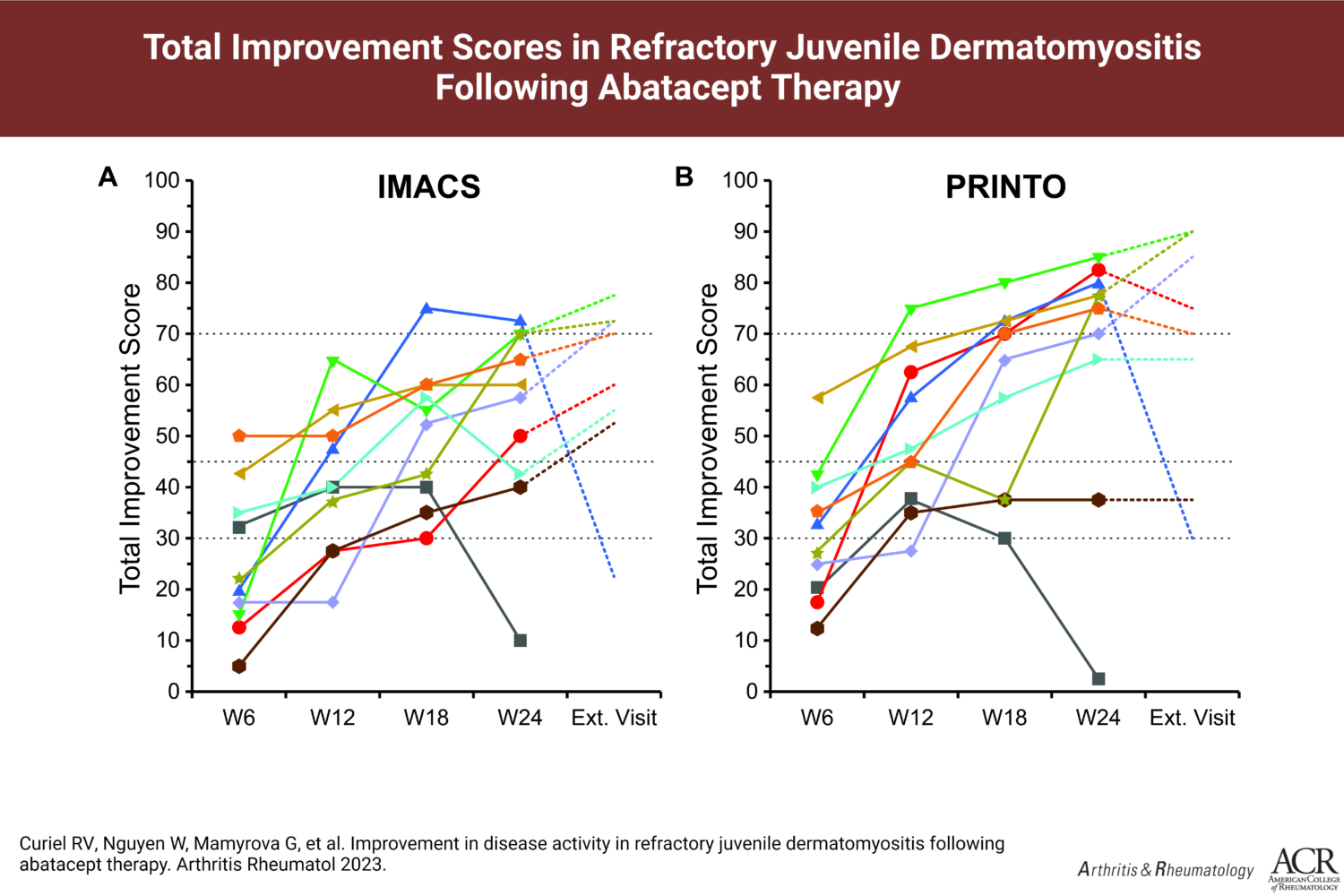
The ten JM patients enrolled in the pilot study were between the ages of seven and 17, and all had moderate disease activity and moderately decreased functional disability. Five of the ten patients met the definition of improvement at week 12 of the trial, and nine met the definition by week 24. At week 24, three of the ten patients saw major improvement, four saw moderate improvement, and two saw minimal improvement when using Core Set Measures against a scale utilized by the International Myositis Assessment and Clinical Studies Group (IMACS).
When assessing improvements in Core Set Measures against a similar scale used by the Pediatric Rheumatology International Trials Organization (PRINTO), seven of the nine improved patients saw major improvements in their scores. One patient was discontinued from the trial due to worsening interstitial lung disease.
“These results are preliminary but are certainly very encouraging,” said Dr. Rodolfo Curiel, the trial’s principal investigator.
“We have much more data to assess before we publish the results, yet the pilot study showed that patients with refractory JDM treated with abatacept resulted in lower disease activity and clinically significant responses in the majority of patients.” Dr. Curiel is the Director of the Cure JM Center of Excellence at George Washington University.