1. What is Cabaletta’s RESET Myositis trial?
RESET-Myositis is a phase 1/2 clinical trial being conducted to evaluate the effects of the investigational cell treatment CABA-201 in patients with dermatomyositis (DM), antisynthetase syndrome (ASyS) and immune-mediated necrotizing myopathy (IMNM) who have active disease.
Patients with juvenile myositis and dermatomyositis are eligible to apply.
2. What is CABA-201?
CABA-201 (CAR-T) is the investigational treatment used in the RESET-Myositis study. This clinical trial evaluates the safety, tolerability and effectiveness of CABA-201 in the treatment of myositis.
CABA-201 is an investigational chimeric antigen receptor (CAR) T cell therapy designed to treat myositis by targeting the B cells that may be causing the disease.
CABA-201 is made from your own T cells, a type of white blood cell, and uses your own immune system to get rid of the disease-causing B cells and to restore healthy B cells in your body.
3. Who is eligible?
Adult Patients who:
- Are 18 to 65 years of age.
- Have a diagnosis of myositis.
- Have active disease despite being on medications to treat myositis.
- Have at least one autoantibody associated with myositis. As part of the study screening process, autoantibody testing would be performed.
Additional requirements will apply. Only a study doctor can determine eligibility to participate in the study.
Juvenile Patients who:
- Are 6 to 17 years of age.
- Have a diagnosis of myositis.
- Have active disease despite being on medications to treat myositis.
- Have at least one autoantibody associated with myositis. As part of the study screening process, autoantibody testing would be performed.
Additional requirements will apply. Only a study doctor can determine eligibility to participate in the study.
4. How do I learn more?
The study team is standing by to answer all questions. Please contact the site nearest you:
- Orange County, UC Irvine. Contact Matthew Douraghi at (714) 456-8122 or douraghm@hs.uci.edu
- Jacksonville, FL, Mayo Clinic Florida. Contact Matthew Dwarika at (904) 953-4511 or dwarika.matthew@mayo.edu
- Chicago, IL, Northwestern Memorial Hospital. Contact Matthew Selle at (312) 472-8398 or matthew.selle@nm.org
- Chicago, IL, The University of Chicago Medical Center. Contact Dr. Iazsmin Ventura, MD at (773) 702-6619 or iventura@uchicago.edu
- Kansas City, University of Kansas Medical Center. Contact Ali Russo at (913) 945-9942 or aciersdorff@kumc.edu
- Rochester, MN, Mayo Clinic. Contact Izzie Meunier at (507) 538-6337 or meunier.Isabelle@mayo.edu
- Portland, OR, Oregon Health and Science University. Contact Kate Lewis at (503) 908-9724 or lewiskat@ohsu.edu
- Nashville, TN, Vanderbilt University Medical Center. Contact Allie Bell at (615) 875-7243 or allie.m.bell@vumc.org
- Houston, TX, Houston Methodist Hospital. Contact Delrose A. Vernon (346) 356-3347 or davernon@houstonmethodist.org
- Houston, TX, University of Texas MD Anderson Cancer Center. Contact Christina Briggs Amos (832) 421-2982 or cbamos@mdanderson.org
- Or to the clinical trial team at (267) 759-3100 or clinicaltrials@cabalettabio.com
Study details are listed at ClinicalTrials.gov.
5. Are juvenile myositis patients eligible?
Yes. This trial is open for myositis patients aged 18-65. This includes juvenile myositis patients who are now aged 18-65.
There is also a juvenile cohort that is enrolling patients aged 6 to 17.
Please view the trial criteria here.
Additional requirements will apply, and only a study doctor can determine eligibility to participate in the study. To find out more, contact the site nearest you.
6. Who decides if I can participate?
The study team will discuss the decision process with you. To find out more, contact the site nearest you.
7. What if I change my mind about participating?
Participation in a clinical trial is voluntary, and participants can withdraw at any time. The trial team and your doctor (whom you normally see for your JM or DM care) will be your best source of information. To find out more, contact the site nearest you.
8. Why participate in this study?
By participating in the RESET-Myositis study, you will:
- Play an important role in understanding myositis.
- Receive study-related care, tests, and procedures from a study doctor at no cost to you.
- Receive compensation for your time during certain study visits and reimbursement for reasonable travel expenses.
With any clinical trial there are risks, that you will need to discuss with your doctor and the clinical trial sites.
9. Are in-person visits required?
Yes, in-person visits will be required. Each site will be able to answer your questions about the expected number of visits and what you can expect at each visit. To find out more, contact the site nearest you.
10. Where is the trial?
Current sites are:
- Orange County, UC Irvine
- Kansas City, University of Kansas Medical Center
- Rochester, MN, Mayo Clinic
- Portland, OR, Oregon Health and Science University
- Nashville, TN, Vanderbilt University Medical Center
Sites involved in the study are listed at ClinicalTrials.gov
11. Is travel reimbursed?
Yes, reimbursement for reasonable travel will be permitted and will be handled by each trial site. To find out more, contact the site nearest you.
12. What are the potential risks of participating in this trial?
Therapy with CAR-T cells may cause serious and potentially life-threatening side effects. The potential risks of participating in this clinical trial will be explained to you before you decide whether to participate.
13. Has CAR-T or CABA-201 been used in myositis before?
Please discuss with the trial site closest to you who will be able to answer all of your questions.
14. Tell me more about clinical trials in general.
Click here to learn about Clinical Trials.
15. Tell me more about CABA-201 and CAR-T.
View the graphic below and please contact your nearest trial site with any questions.
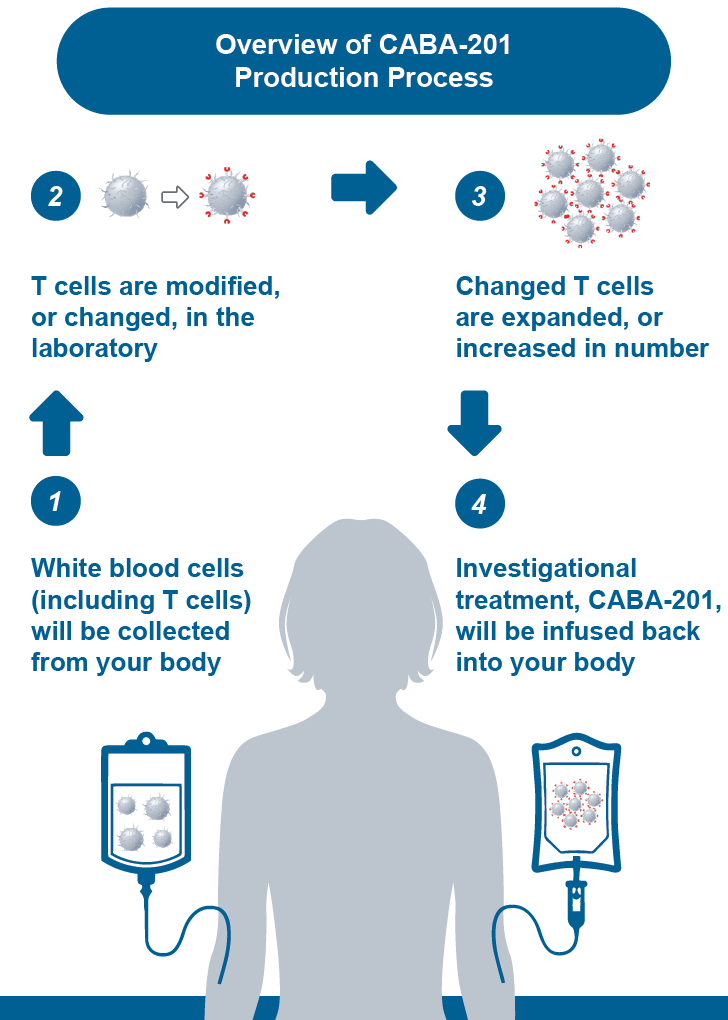
Image courtesy of Cabaletta Bio’s website regarding the CABA-201 process.