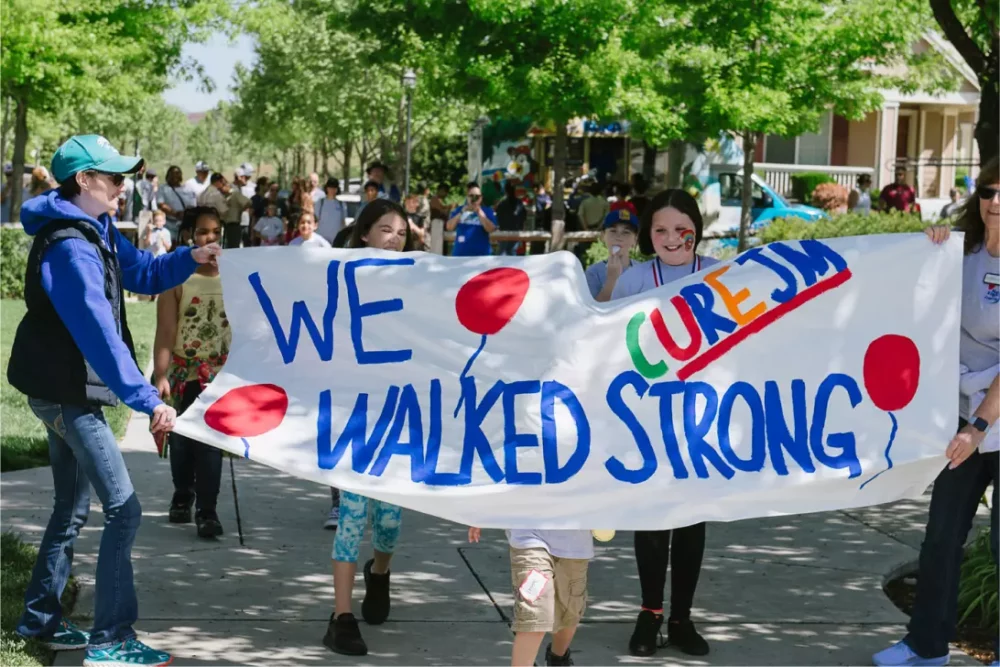Cure JM Events
Get involved with Cure JM throughout the year! Participating in events is a great way to move the mission forward and meet other JM families.

2024 Walk Strong to Cure JM
Cure JM families and friends are joining forces to Walk Strong together. Join the hundreds of Cure JM families who have taken steps toward better treatments and a cure.
To join a walk near you, click below! Or start a walk in your area!
Town Halls
Educational Family Events
Meet other Cure JM families at an upcoming educational event, family day, or conference in your area.
2023 National Family Conference
The Cure JM Family Conference was a success! Families from around the country gathered to learn about the latest treatment and research advances to share in our hope and progress.
Click below to read a recap of what happened at the 2023 conference!
Also, stayed tuned for another National Family Conference coming in 2025!